how many valence electrons in titanium|Determine valence electrons using the periodic table : Bacolod The ability of one atom of an element to join another atom during the formation of a molecule is called valency(valence). There are some rules for diagnosing valency. The . Tingnan ang higit pa If you want to be a member of this MABUHAY City group.. You must be a resident of Mabuhay City subdivision.. Paliparan 3.Dasmariñas City Cavite. OR MESSAGE PM,to Admin o Moderator to approve ur.PCSO Lotto Result Today - PCSO announces complete lotto results for Thursday, January 18, 2024. Monitor here for 6/42, 6/49, EZ2 (2D Lotto), Swertres (3D Lotto) and 6D Lotto winning numbers.
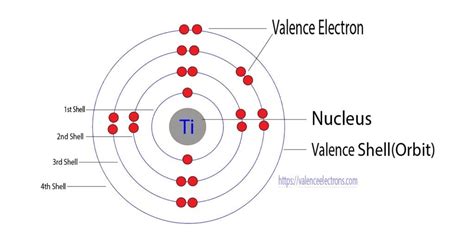
how many valence electrons in titanium,Titanium (Ti) is an element located in Group 4 of the periodic table. The number of valence electrons an element has is determined by its position in the periodic table. In the case of titanium, it has an atomic number of 22, which means it has 22 electrons. The electronic configuration of titanium is 1s2 2s2 2p6 . Tingnan ang higit pa
The 1st element in group-4 is titanium. The elements in groups 3-12 are called transition elements. The valence electrons are the . Tingnan ang higit pa
The valence electrons have to be determined by following a few steps. The electron configuration is one of them. It is not . Tingnan ang higit pa
The elements that have 1, 2, or 3 electrons in the last shell donate the electrons in the last shell during bond formation. The elements . Tingnan ang higit paThe ability of one atom of an element to join another atom during the formation of a molecule is called valency(valence). There are some rules for diagnosing valency. The . Tingnan ang higit pa
Mar 23, 2023 To find the number of valence electrons for Titanium (Ti) we need to look at its electron configuration. This is necessary because Ti is a transition metal (d block element) and we need to take. Anne Marie Helmenstine, Ph.D. Updated on May 19, 2024. You may assume . How many valence electrons does Titanium have? Titanium is undoubtedly one of the strongest chemical elements in the world. It is a pure metal with a solid grey composition and low density.Valence electrons: The electrons present in the outermost shell of an atom are known as the valence electrons. Titanium Ti: Titanium is a transition metal that belongs to the d .The number of valence electrons determines most of an atom's chemical behaviors. So, it's important to be able to identify how many valence electrons atoms of different .The atomic number of each element increases by one, reading from left to right. BlockElements are organised into blocks by the orbital type in which the outer electrons .

Therefore, the number of electrons in neutral atom of Titanium is 22. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the atom.
There are 4 Valence electrons in the outer shell of Titanium. Titanium Number of Valence Electrons. Titanium has 4 valence electrons in its outer shell. Period Table.Valence electrons: The electrons present in the outermost shell of an atom are known as the valence electrons. Titanium Ti: Titanium is a transition metal that belongs to the d-block element. The atomic number of Titanium is 22. The electronic configuration of Titanium is Ar 3 d 2 4 s 2.
View this answer. Answer: 4 valence electrons. Titanium is a transition metal with an electron configuration of [ A r] 4 s 2 3 d 2. Electrons. See full answer below.Determine valence electrons using the periodic table Titanium has four valence electrons. The nuclear number of titanium is 22 22 and has a place with the progress metal gathering. Note: We can write the total electron configuration for titanium is as per the following: 1s22s22p63s23p63d24s2 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 2 4 s 2. For progress metals, and specifically those in sections.
how many valence electrons in titanium Titanium’s atomic symbol is Ti, and its atomic number is 22. It belongs to the transition metal group and can form compounds at an oxidation state of +4. Titanium’s abbreviated electron configuration is [Ar] 3d 2 4s 2. This shows which valence electrons can be lost in a bond with other elements. This table of element valences includes the maximum valence and most common valence values in chemistry. . (columns) of the periodic table. While these are the most common valences, the real behavior of electrons is less simple. Remember an element's electron cloud will become more stable by filling, emptying, or half-filling the .Contributors and Attributions. 3.10: Valence Electrons is shared under a CC BY-NC license and was authored, remixed, and/or curated by LibreTexts. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s sublevel are called inner-shell electrons ..The full electron configuration of titanium is 1s2 2s2 2p6 3s2 3p6 4s2 3d2 and the abbreviated electron configuration is [Ar]3d24s2. With the Electron configuration of each element, it is possible to specify how the electrons are structured in the atoms of this element. In the case of titanium, its average radius is 140 pm, its covalent radius . Solution. Element A is located in Period 2, the 5th position in 2p-block.Before the electrons are placed in 2p subshell, the 2s subshell must be filled first. This means that A has two valence electrons in 2s (2s 2) and five valence electrons in 2p (2p 5).Answer: 2s 2 2p 5. It has 2 + 5 = 7 valence electrons.. Element B is located in Period 3, the 2nd . Introduction. T here are four principle orbitals (s, p, d, and f) which are filled according to the energy level and valence electrons of the element. All four orbitals can hold different number of electrons. The s-orbital can hold 2 electrons, and the other three orbitals can hold up to 6, 10, and 14 electrons, respectively.Electron configuration The arrangements of electrons above the last (closed shell) noble gas. . so it doesn't corrode. Its density is 4.5 grams per cm3, much less than iron, so titanium alloys are important in the aerospace industry. It was used to make much of the SR-71 Blackbird, the world's fastest manned aircraft, as well as a major parts .
The electron configuration for Titanium ion (Ti 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6. The number of valence electrons available for the Titanium atom is 4. Titanium is situated in the transition metal group .
Each aluminum has three valence sites, while each chlorine has only one, and so it requires three chlorine atoms to satisfy one aluminum, and the formula is AlCl 3 . b) Again chlorine has a valence of 1. Phosphorus is in group V and might have a valence of 5 or of 8 – 5 = 3. Therefore we predict formulas PCl 5 or PCl 3.Strengths. The Bohr model represents the particle nature of electrons. So, it's easy to see that the atom above contains two electrons. As we'll discuss later in the article, atomic electrons exist at specific energy levels. The Bohr .
Valence electrons. Electron configurations of ions. Electron configurations of the 3d transition metals. Atomic structure and electron configuration . so the next element in the .And so for this video, we're only talking about the valence electrons for elements in the main groups. When we talk about the main groups, you're using the one through eight system for classifying groups. So one, two, three, four, five, six, seven, and eight. So we're going to ignore the other way to number the groups.
2. Find the electron configuration for the element you are examining. Once you know an element's electron configuration, finding its number of valence electrons is quite simple (except, of course, for the transition metals.) If you're given the configuration from the get-go, you can skip to the next step.

Titanium is a chemical element of the periodic table with chemical symbol Ti and atomic number 22 with an atomic weight of 47.8671 u and is classed as a transition metal. . Valence electrons : 4: Valency electrons : 2,3,4: Bohr model: Electron shell for Titanium, created by Injosoft AB Ti. Figure: Shell diagram of Titanium (Ti) atom. Orbital . Answer link. "Ti"^ (2+): ["Ar"]3d^2 A good place to start when trying to figure out the electron configuration of an ion is the electron configuration of the neutral parent atom. In this case, titanium, "Ti", is located in period 4, group 4 of the periodic table and has an atomic number of 22. This means that a neutral titanium atom will .
how many valence electrons in titanium|Determine valence electrons using the periodic table
PH0 · What are the valence electrons of titanium? Chemistry Question
PH1 · What are the valence electrons of titanium? Chemistry Question
PH2 · Valences of the Chemical Elements
PH3 · Valence Electrons Chart for All Elements
PH4 · Titanium Valence Electrons
PH5 · Titanium Electron Configuration (Ti) with Orbital
PH6 · Titanium
PH7 · How to Find the Valence Electrons for Titanium (Ti)?
PH8 · How to Find the Valence Electrons for Titanium (Ti)
PH9 · How Many Valence Electrons Does Titanium (Ti) Have?
PH10 · Determine valence electrons using the periodic table